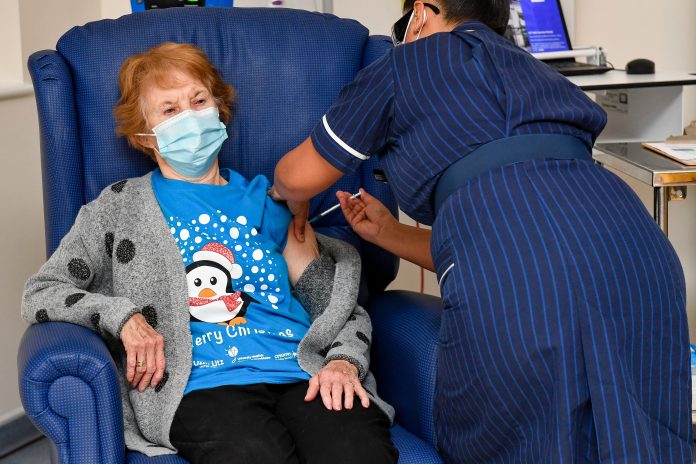
A British grandmother has become the first person in the world to receive the Pfizer COVID-19 vaccine outside of a trial following its rapid clinical approval.
Britain has begun the mass-vaccination of its population against COVID-19, becoming the first Western nation to do so in a global endeavour that poses one of the biggest logistical challenges in peacetime history.
On a day dubbed “V-Day”, health workers on Tuesday started inoculating people with a shot developed by Pfizer and BioNTech, with the country a test case for the world as it contends with distributing a compound that must be stored at -70C.
Margaret Keenan, a grandmother who turns 91 in a week, became the first person in the world to receive the vaccine outside of a trial when she received the shot at her local hospital in Coventry, central England.
“It’s the best early birthday present I could wish for because it means I can finally look forward to spending time with my family and friends in the New Year after being on my own for most of the year,” she said.
The launch will fuel hope that the world may be turning a corner in the fight against a pandemic that has killed more than 1.5 million people, with Britain the worst-hit European country with over 61,000 deaths.
Britain is the first nation globally to begin mass inoculations with the Pfizer-BioNTech shot, one of three vaccines that have reported successful results from large trials after being developed in record time.
Health Secretary Matt Hancock described the start of vaccinations as “V-Day”.
“If we manage to do that for everybody who is vulnerable to this disease, then we can move on and we can return to normal,” he said, adding that he expected millions to be vaccinated by the end of the year.
The country has ordered enough supplies of the Pfizer-BioNTech shot to vaccinate 20 million people. The developers said it was 95 per cent effective in preventing illness in final-stage trials.
Russia and China have both already started giving domestically produced vaccine candidates to their populations, though before final safety and efficacy trials have been completed.
In Britain, about 800,000 doses are expected to be available within the first week, with care home residents and carers, the over-80s and some health service workers prioritised.
“Thank you to our NHS, to all of the scientists who worked so hard to develop this vaccine, to all the volunteers – and to everyone who has been following the rules to protect others,” Prime Minister Boris Johnson said on Twitter.
“We will beat this together.”
The logistical challenges in distributing the vaccine, which only lasts five days in a regular fridge, mean it will first go to dozens of hospitals and cannot yet be taken into care homes.
Transport and distribution could prove more challenging in bigger countries, including the US and India which have been worst-hit by the pandemic, and in hotter nations.
Another vaccine to have had trial success, developed by AstraZeneca and Oxford University, is viewed as offering one of the best hopes for many developing countries because it is cheaper and can be transported at normal fridge temperatures.
Late-stage trials found it had an average success rate of 70 per cent.
Britain hopes for regulatory approval of that vaccine in the next couple of weeks.
In total Britain has ordered 40 million doses of the Pfizer/BioNTech shot. As each person requires two doses, that is enough to vaccinate 20 million people in the country of 67 million.
But the country is spreading it bets and has ordered 357 million doses of seven different COVID-19 vaccines in all.