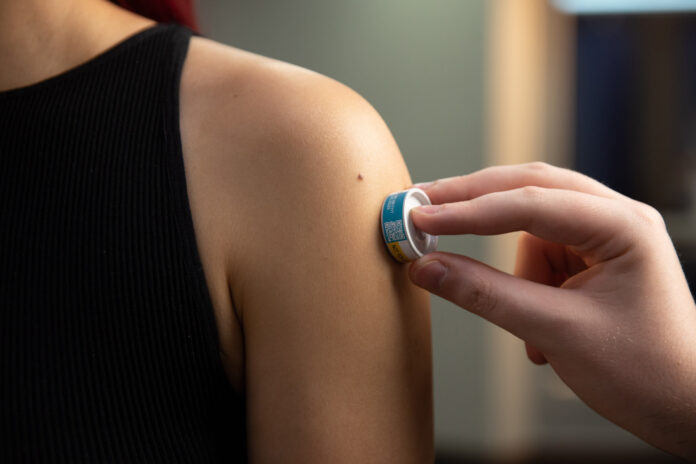
Researchers are closer to delivering a needle-free vaccine for COVID-19, through a promising trial at the University of the Sunshine Coast.
Biotech company Vaxxas on Wednesday announced interim results from the first phase of the clinical trial, which involves high-density microarray patch (HD-MAP) technology.
The patch is based upon HD-MAP delivery of a vaccine candidate from the University of Texas at Austin (UTA).
The vaccine candidate is a second-generation version of the spike protein used in major COVID-19 vaccines and has been modified for stability and immunogenic response, giving potential coverage of all known SARS-CoV-2 variants.
Interim data from the study, which started in 2021, showed the patches were well tolerated, with no serious or severe adverse events.
Analysis of samples showed the vaccine increased relevant antibody levels by eight-fold on average.
Vaxxas chief executive officer David L. Hoey said there were promising signs.
“We are very encouraged by the compelling early data and rapid progress of our needle-free COVID-19 vaccine candidate,” he said.

“We believe our patch-based delivery of a next-generation spike protein has the potential to offer best-in-class protection against COVID-19, along with cost-effective distribution without the need for extensive refrigeration.”
The first phase of the clinical trial assessed the safety, tolerability and immunogenicity of the patch in 44 healthy adults, aged 18 to 50.
Participants were required to have had three doses of an authorised COVID-19 vaccine prior to enrolment in the study, with the last dose received at least four months prior to participating.
The interim data supported Vaxxas’ progress towards seeking approvals for a COVID-19 vaccine patch with the Therapeutic Goods Administration in Australia and Food and Drug Administration in the United States.
With successful completion of the first phase, and following subsequent phase two and phase three studies, the COVID-19 vaccine patch could be available as early as 2025.
Pre-clinical research published in Science Advances and Vaccine, undertaken with the University of Queensland and other collaborators, demonstrated that the UTA COVID-19 vaccine candidate delivered using Vaxxas’ HD-MAP resulted in enhanced virus neutralising antibody and T-cell responses against all major variants of concern, including alpha, beta, gamma, delta and omicron, when compared to needle and syringe vaccination.
Scroll down to SUBSCRIBE for our FREE news feed, direct to your inbox daily.