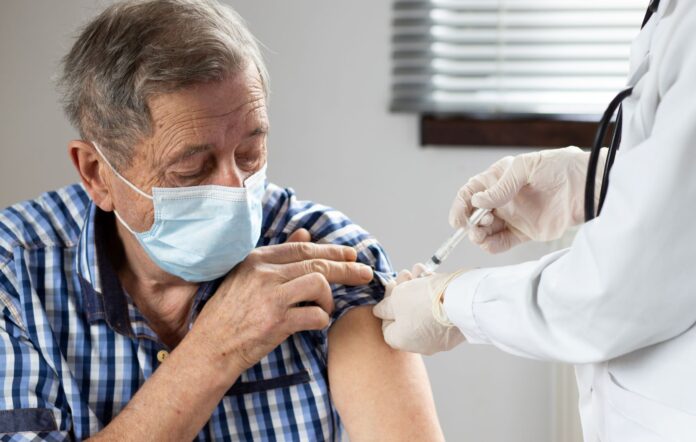
The University of the Sunshine Coast is partnering with an Australian biotechnology company to investigate needle-free technology for administering influenza vaccines.
The study will look at the safety of a vaccine delivered using the high-density microarray patch (HD-MAP) developed by Vaxxas assess whether it provides an immune response comparable to a standard dose delivered by a needle.
The research will be led by Dr Nischal Sahai from UniSC Clinical Trials.
“It’s very exciting to be working with innovative Australian vaccination technology that could potentially change the way vaccines are administered around the world,” Dr Sahai said.
“HD-MAP technology could mean that vaccines no longer need to be refrigerated, making it easier to transport them safely to people in remote locations.”
The study requires healthy volunteers aged 18-50 who are in good general health and have a body mass index within the range of 18-32.
Participants will be required to visit the UniSC Clinical Trials clinics at Sippy Downs or South Bank in Brisbane about six times over two-and-a-half months.
Vaxxas chief technology officer Dr Angus Forster said he was excited to again work with UniSC Clinical Trials after running a similar study in 2021 for measles and rubella and currently for COVID-19.
“We are pleased to be partnering again with the team at UniSC Clinical Trials to undertake a phase one study of an influenza vaccine using our HD-MAP technology,” Dr Forster said.
“We hope to demonstrate that delivering an influenza vaccine using our HD-MAP technology can potentially be just as effective as the traditional intramuscular vaccine needle injection.”
Influenza is a common infectious disease that is responsible for three to five million severe cases worldwide annually, along with up to 650,000 deaths per year.
Vaxxas chief executive officer David L. Hoey said the technology had the potential to increase vaccination coverage and uptake.
“Vaccine delivered via HD-MAP patch technology removes the need for needle and syringe, is easy to use, and can potentially be self-administered. It also has the potential to simplify distribution by removing or reducing the need for refrigeration,” he said.
“The potential benefits of Vaxxas’ needle-free vaccine platform, supported by clinical trials, are all factors that could improve access to, and acceptability of, current influenza vaccines. This will offer greater protection each season to communities in Australia and around the globe.”
Researchers estimate that fear of needles affects up to 25 per cent of adults and may lead to 16 per cent of people in developed countries skipping vaccinations.
Those interested in participating can find more information here.
SUBSCRIBE here now for our FREE news feed, direct to your inbox daily.